iRecist and Tumor Response to Immunotherapy
When immunotherapy is used to treat cancer, clinicians often see effects that are different from those observed following treatment with surgery, radiation, or chemotherapy. For example, tumor response can be delayed or patients may show 'pseudoprogression,' an inflammation around the tumor caused by the stimulation of the immune system that can be mistakenly interpreted in imaging as progression. Also, radiologists employed in immunotherapy trials may see a wide variety of radiologic manifestations due to immune-related adverse events—colitis, hepatitis, hypophysitis, thyroid enlargement, pneumonitis, or sarcoid-like reactions (lymphadenopathy and pulmonary granulomatosis), to name a few.
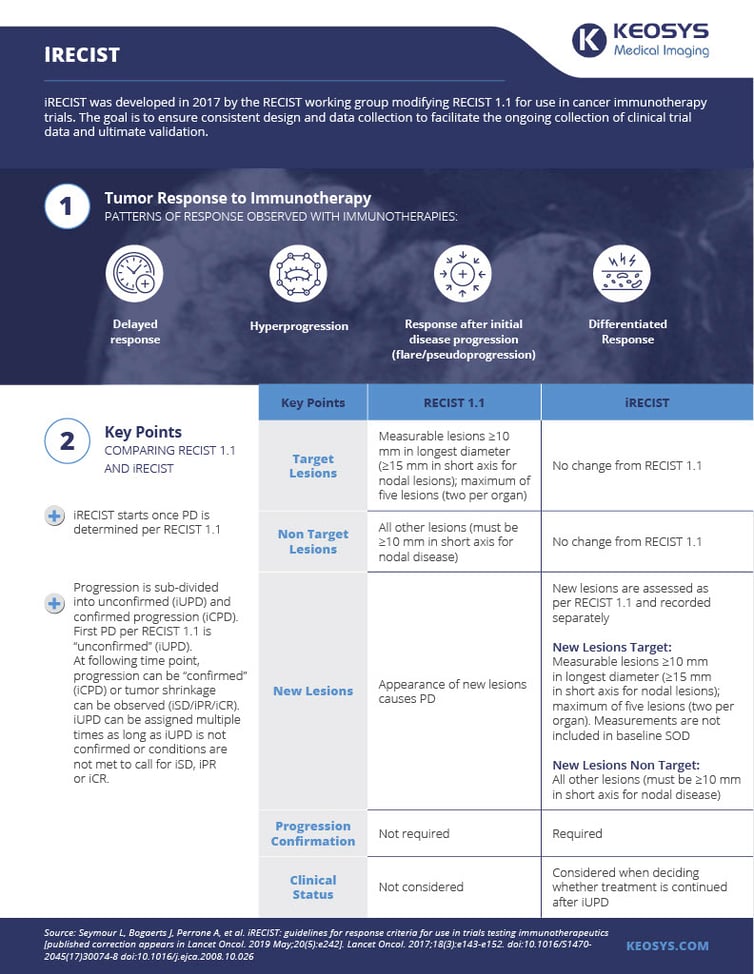
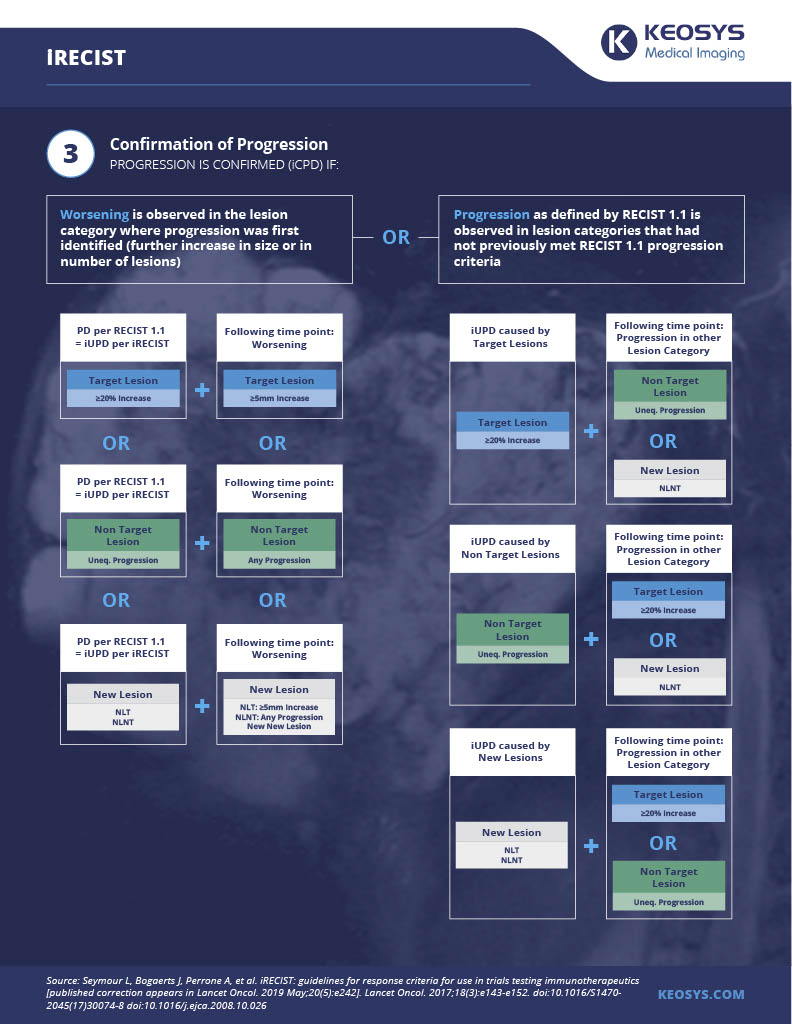
As a result of phenomena like these, the RECIST working group in 2017 developed iRECIST, a modification of RECIST 1.1 designed specifically for use in cancer immunotherapy trials.
We at KEOSYS believe iRECIST deserves careful examination and, as a result, we have prepared the following infographic on the topic. We hope you’ll find it interesting and useful.
Below is our infographic on iRECIST criteria. You can download it here.
Keep watching the Keosys newsletter as we'll have more infographics on additional topics coming soon.