This is an excerpt from our free eBook “Imaging and Read Criteria in Cancer Immunotherapy Clinical Trials". To access the full eBook, click here.
iRECIST is the most used immune-based read criteria. However, as iRECIST still requires validation, it is often used for exploratory analysis in addition to RECIST 1.1 assessments.
iRECIST follows recommendations similar to those of RECIST 1.1 on a lesion level in terms of methods of measurement (unidimensional), categorization (Target Lesions, Non-Target Lesions, New Lesions) and size criteria (up to 5 Target Lesions >10mm, maximum 2 per organ). The methodology followed to determine overall response is also comparable between iRECIST and RECIST 1.1.
However, iRECIST takes into account the following concepts:
- “Resetting the bar”: When RECIST 1.1 progression is followed at the next assessment by tumor shrinkage, the bar is reset (instead of staying in PD as per RECIST 1.1)
- “Worsening”: When further progression is observed in the lesion category that triggered an initial PD per RECIST 1.1, the worsening of the lesion category confirms progression. This follows the Bayesian inference method in which a bit more increase in size is further evidence of progression.
Additionally, when new lesions appear with iRECIST, instead of calling for PD as per RECIST 1.1, they are categorized as New Lesions Target (NLT) or New Lesions Non-Target (NLNT) and are monitored at subsequent time points (quantitatively for NLT and qualitatively for NLNT).
To distinguish between response according to RECIST 1.1 and iRECIST, a specific lexicon has been developed with the overall response of progression sub-divided into unconfirmed and confirmed progression:
iRECIST Lexicon
| iCR | Immune Complete Response |
| iPR | Immune Partial Response |
| iSD | Immune Stable Disease |
| iUPD | Immune Unconfirmed Progression |
| iCPD | Immune Confirmed Progression |
In Practice:
iRECIST guidelines start once progression is determined per RECIST 1.1. Instead of PD, overall response is categorized as iUPD.
Progression is confirmed (iCPD) at the following time point if:
- Worsening is observed in the lesion category where progression was first identified (further increase in size or in number of lesions)
or - Progression as defined by RECIST 1.1 is observed in lesion categories that had not previously met RECIST 1.1 progression criteria
Confirming Progression
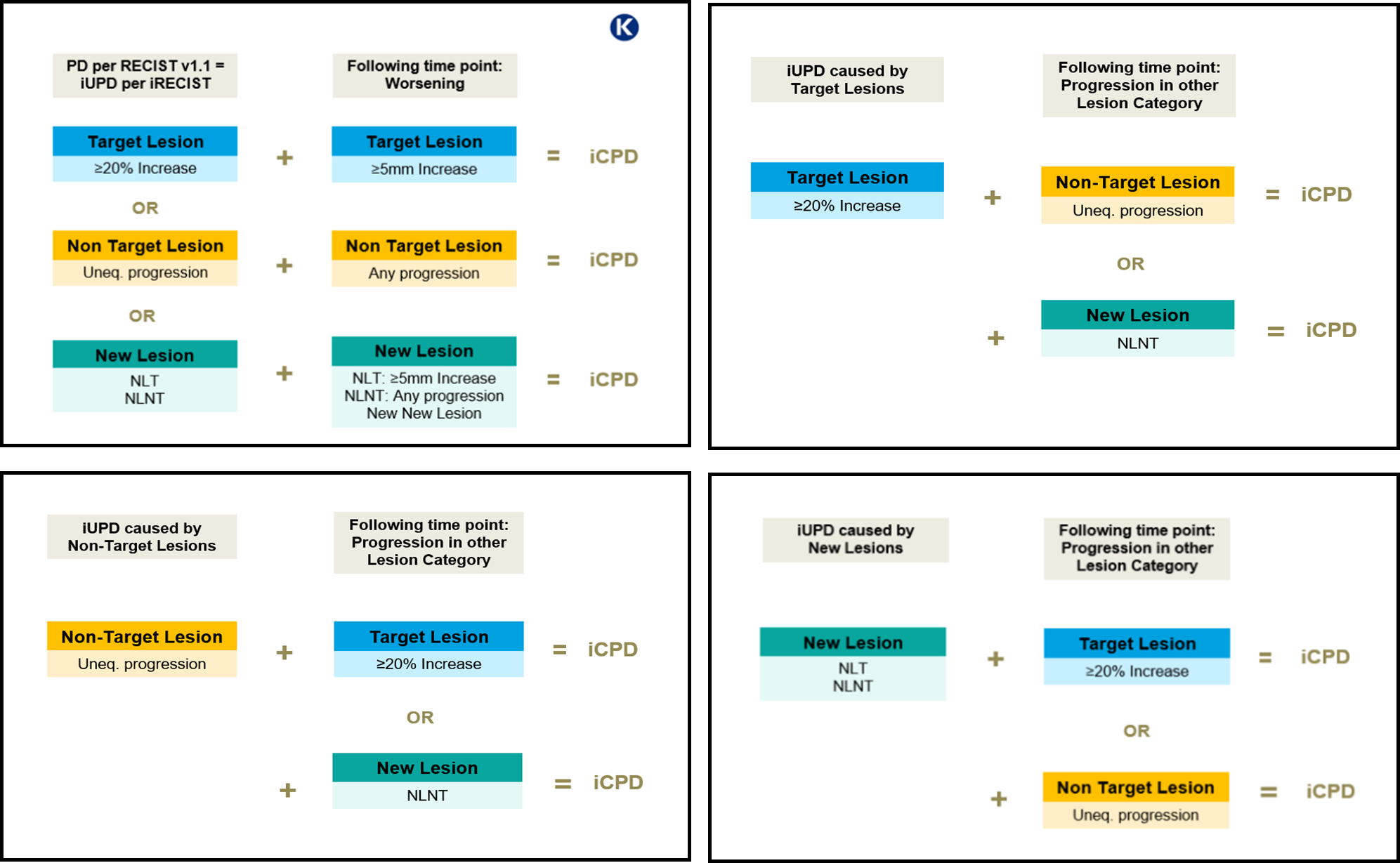
Uneq., unequivocal; NLT, New Lesion Target; NLNT, New Lesion Non-Target; iUPD, unconfirmed progressive disease; iCPD, confirmed progressive disease
The Bar Is Reset If:
Compared to baseline tumor, shrinkage is observed that meets the criteria of iCR, iPR or iSD.
Target Lesions weigh more heavily than Non-Target Lesions or New Lesions. iUPD resolves to iSD or iPR when:
- No drivers or iCPD are observed (i.e., no new cause of PD or worsening of any existing cause)
- Target Lesions are below the PD threshold whether they were or were not PD at the initial iUPD scan even if New Lesions are still present or Non-Target Lesions have not reduced in size
Tumor Shrinkage
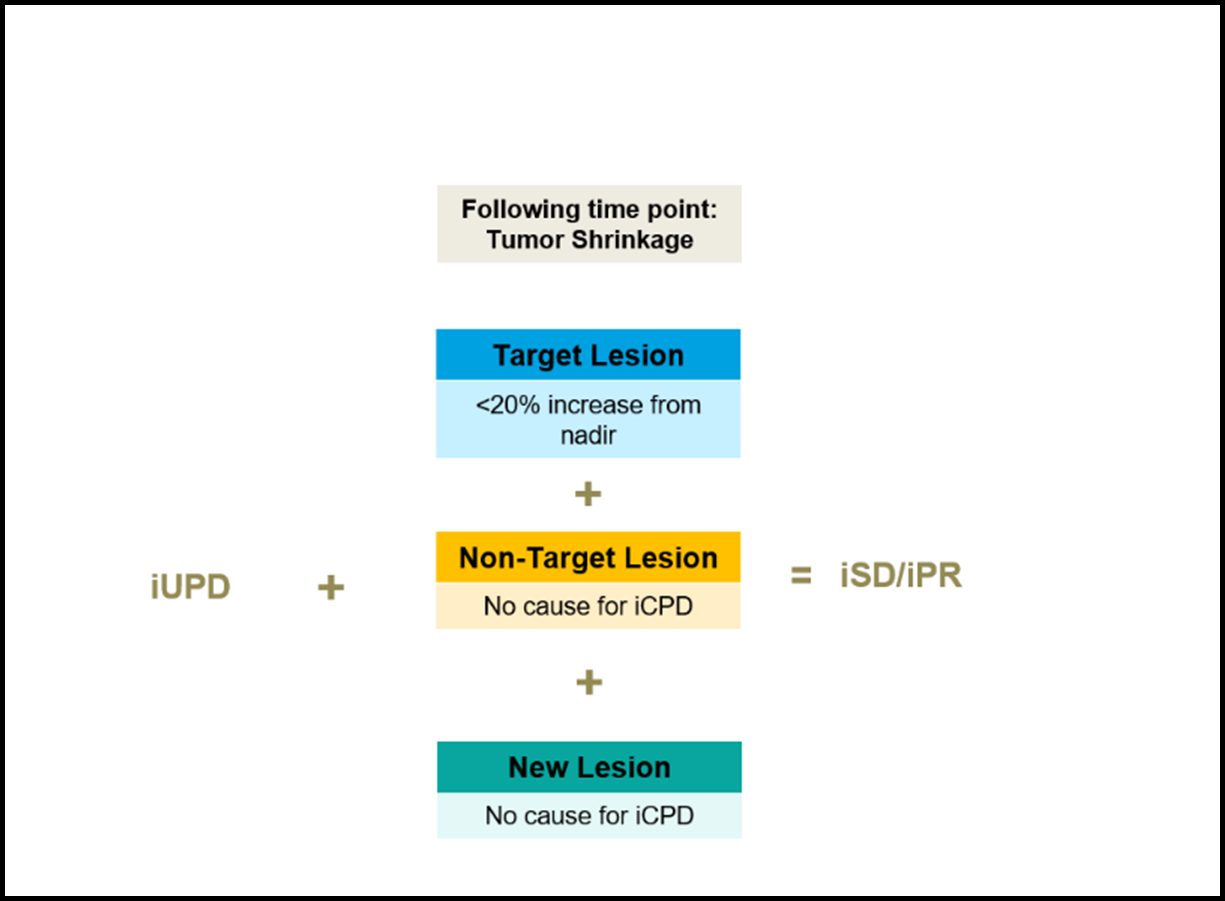
iUPD, unconfirmed progressive disease; iCPD, confirmed progressive disease; iSD, immune stable disease; iPR, immune partial response
iUPD must occur again (compared to nadir values) and then be confirmed at the next assessment for iCPD to be assigned. iUPD can be assigned multiple times as long as iUPD is not confirmed or conditions are not met to call for iSD, iPR or iCR.
Overall Response Considerations:
- Response for each lesion category
- Response for each lesion category at the previous time point
- Overall response at the previous time point
The table below summarizes the rules for deriving overall response.
Overall Time Point Response per iRECIST
-10.jpg?width=1267&name=KEO052-Immunotherapies%20-Final-BrackenEdit-2021May12%20(1)-10.jpg)