Medical imaging is an essential component for clinical drug development from early phases to registration. It can be used to determine the clinical effect of a drug, and this information can then be used to expedite a drug's regulatory approval. In oncology, a decrease (or stabilization) in tumor size, as evidenced by imaging, has been used as the primary endpoint in several recent clinical trials that led to FDA approval. And medical imaging has even become a gold standard for pharmaceutical companies and health authorities.
It’s not just oncology that uses imaging to determine clinical endpoints. Imaging has a key role in trials for cardiovascular disease and Alzheimer's disease, to name just two. But since oncology represents the largest growth area for imaging-based endpoints, it forms the basis of this blog post. We’ll look at selected drugs approved in 2015–2018 on the basis of imaging results.
The Importance of Standardization
It’s important to have standardized response criteria for acquiring and interpreting image-based endpoints, as noted in FDA guidance. Any variability may reduce a clinical trial’s ability to detect drug treatment effects.
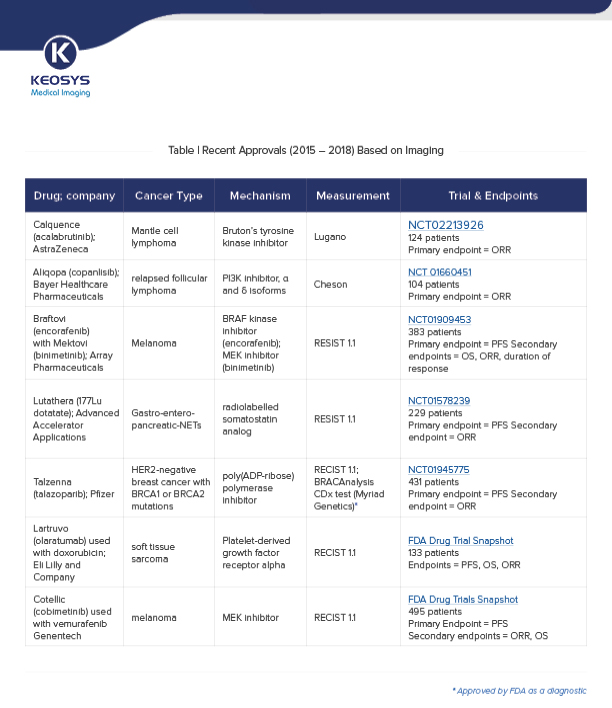
There are several criteria used in recent approvals (see table) to ensure standardization:
RECIST1.1 (Response Evaluation Criteria in Solid Tumors) is a widely used criteria to standardize the measurement of tumor size and tumor response. It includes definitions of minimum size of measurable lesions and how many lesions to follow, and relates to tumor measurements using radiographic imaging such as CT and MRI.
RECIST cannot be used for certain drugs, targets or combinations — such as tyrosine kinase inhibitors in lymphoma — that stabilize disease without causing tumor shrinkage. The Cheson and Lugano guidelines advise on response assessment in non-Hodgkin’s and Hodgkin’s lymphoma using PET/CT in addition to CT/MRI (except for FDG-avid lymphoma)
Read more about imaging criteria in our recent blog post.
Let's next look at how imaging is used in oncology clinical trials. It can be used to measure several key endpoints that are used in the drug approval process.
One endpoint widely used in clinical trials is progression-free survival (PFS). This is the time from randomization until disease progression or death. Compared to survival studies, PFS endpoint requires fewer patients and a shorter follow-up. Imaging-based tumor measurements on CT or MRI and/or the appearance of new lesions on a variety of imaging modalities including PET are key determinants for assessing PFS in clinical trials.
Imaging might also be used to assess the proportion of patients with a pre-defined reduction in tumor size; this is known as the objective response rate (ORR). ORR can be directly correlated with the effect of the drug. And importantly, it can be assessed in single-arm trials, meaning fewer patients are needed and the trial can be completed quickly. This is in contrast to the endpoint of overall survival (OS), which must be evaluated in randomized trials.
ORR endpoints are valid surrogates for clinical benefit, since drugs that shrink tumors can be expected (albeit imperfectly) to improve the chance of overall survival (OS) or progression-free survival (PFS). However, the FDA requires follow-up confirmatory data that the drug produces a survival benefit in order to be given continued approval.
Of note, imaging (to asses PFS) enabled the recent approval of Adcetris (brentuximab vedotin) for the treatment of peripheral T-cell lymphoma (PTCL) under the FDA's Real-Time Oncology Review Pilot Program. This program enabled the FDA to approve Adcetris within two weeks of submission.
The table below highlights selected drug approvals based on clinical trials that used imaging to assess PFS and/or ORR. (Click here or on the table for an enlarged version.)
Getting Drugs to Patients Quicker
New applications of imaging in clinical trials continue to emerge. For example, large imaging data sets combined with artificial intelligence and data on clinical outcomes (known as radiomics) aid diagnosis, predict safety and treatment outcomes - thus further limiting the number of subjects that need to be included in a clinical trial. There are still challenges surrounding the use of imaging in clinical trials overcome. For example, imaging design is often tedious and not always adapted to clinical routine. And there’s ongoing debate and active research on how imaging-based surrogate endpoints correlate with OS in oncology.
Nevertheless, let's finish by summarizing the impact that imaging has on drug approvals:
- In the last several years, the FDA has approved several novel and important drugs based on imaging results.
- PFS and ORR surrogate endpoints are accepted by the FDA as a marker of efficacy for oncology drugs.
- Endpoints assessing tumor growth can be evaluated more quickly than survival-based clinical benefit endpoints.
- Imaging is a powerful tool in approval trials as it facilitates the patient access to drugs sooner.