Medical imaging has been used in clinical trials to assess tumor response for 40 years. However, it wasn’t until the US FDA's Modernization Act of 1997 that imaging data could be included in regulatory submissions (as a primary endpoint, and with some conditions). Then in 2004, the FDA's Critical Path Initiative recognized the need to more widely incorporate scientific advances, including advanced imaging technologies, into the drug development process. The FDA guidance on clinical trial imaging endpoints released in 2011 describes standards used today to optimize the quality of imaging data obtained in clinical trials.
The Road to RECIST
As highlighted in the FDA guidance, it’s important to have standardized medical imaging criteria to capture the response of a tumor to an oncology treatment.
The World Health Organization published the first standardized tumor response criteria in 1979 and these were widely used. Nevertheless, several issues arose with the WHO criteria. These included variations in the size and number of lesions to be recorded and ambiguous definitions of progressive disease. In addition, the WHO criteria didn't take into account newer technologies such as MRI.
So in the mid-1990s, an international working party — lead by EORTC — convened, resulting in the publication of new guidelines, RECIST (Response Evaluation Criteria in Solid Tumors) in 2000. RECIST offered a model by which a combined assessment of all lesions, characterized by target lesions (to be measured) and non-target lesions, is used to extrapolate an overall response to treatment. It defined the minimum size of measurable lesions and how many lesions to follow.
In 2009, major revisions were made (resulting in RECIST 1.1), including a reduction in the number of assessed lesions, a new method to classify lymph nodes, clarifications related to a complete response (CR) or partial response (PR) and new methods for measuring disease progression. Of note, RECIST 1.1 states that the finding of a new lesion should be unequivocal (i.e. not attributable to differences in scanning technique or change in imaging modality etc.); this is important as new lesions define progressive disease. RECIST1.1 is today the gold standard for evaluating response in solid tumors.
Incorporating RECIST into Imaging Trials
How are RESCIST 1.1 and related criteria incorporated into clinical trials? Image analysis software and centralized reads are increasingly becoming an important part of capturing the response of a tumor to a treatment – whether it be a chemotherapy or an immunotherapy. Specialized imaging CROs have an increasing role here. These processes all help ensure all images are read uniformly and consistently, minimizing inter-/intra-reader variability and the potential for bias and imaging-related queries.
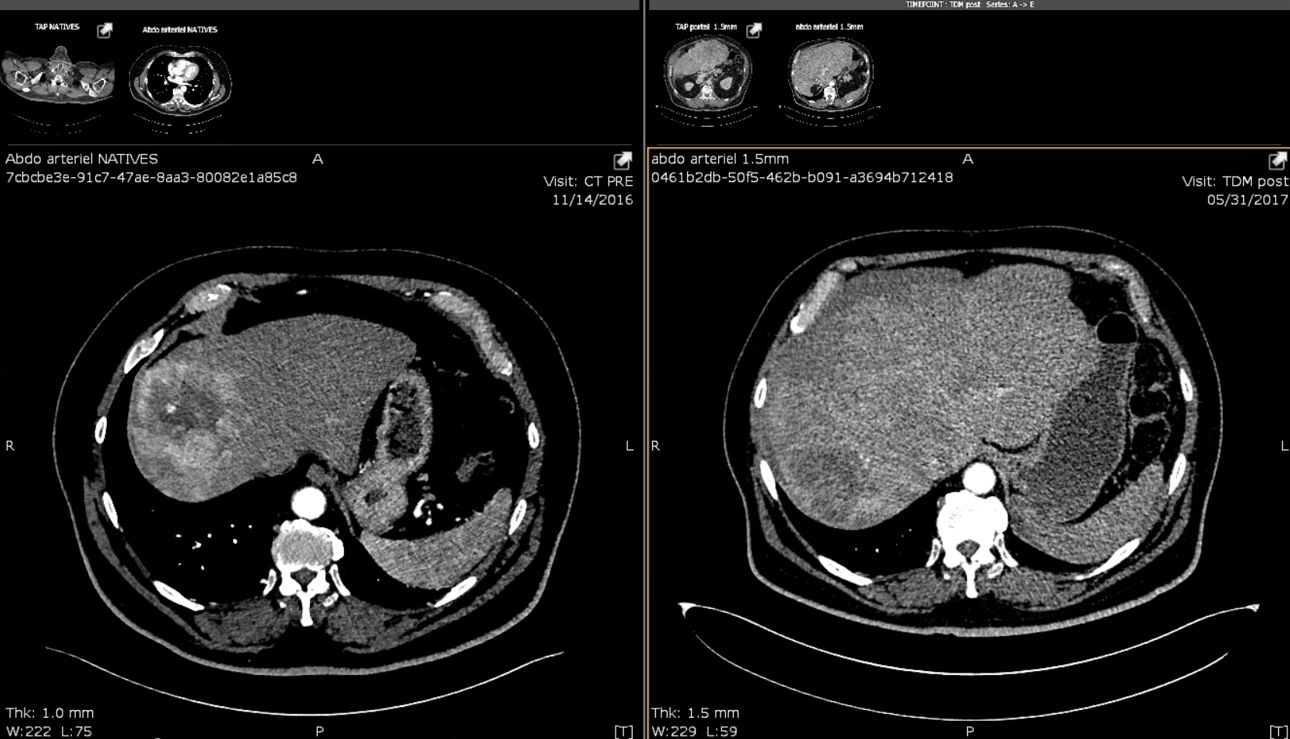
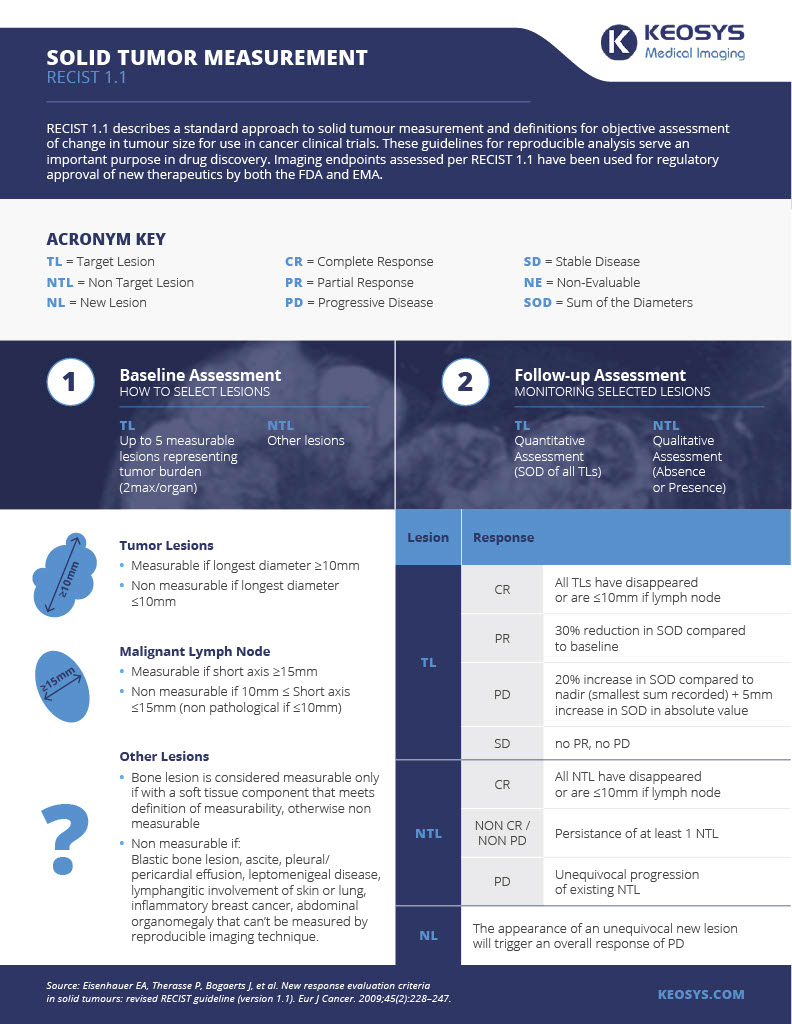
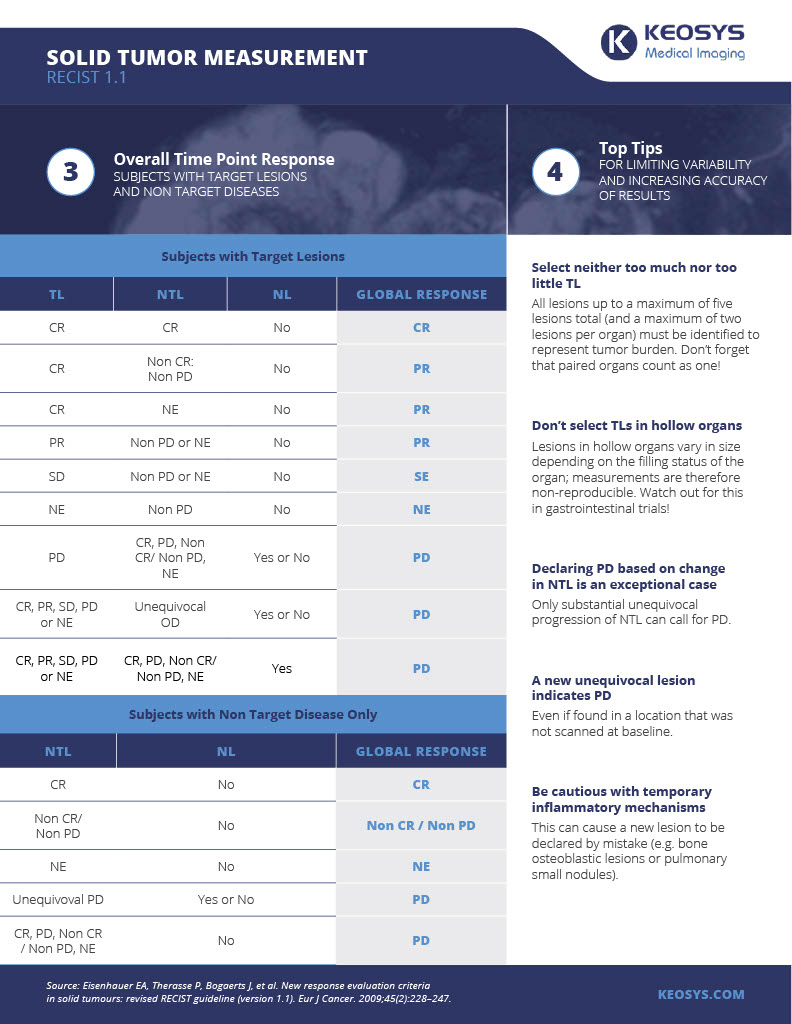
Below is our infographic on RECIST criteria, and how it's used to measure solid tumors in oncology research. You can download it here.
Keep watching the Keosys newsletter as we'll have more infographics on additional topics coming soon.