This is an excerpt from Keosys' free eBook, "The Non-Expert's Guide to Clinical Imaging." To access the full eBook, click here.
One of the key considerations when executing imaging in a trial is the choice of read design. That is, the number, type of readers and the setup you will use to capture and interpret the imaging data.
As trials can have images from a variety of imaging modalities that require review by expert clinical readers (e.g. radiologists, pathologists, cardiologists), one of the greatest challenges is reducing variability in image capture and analysis.
Trial sponsors and CROs must put processes in place to ensure that:
- Initial image acquisition should involve adequately trained people who acquire images according to Image Acquisition Guidelines that help standardize image acquisition across hundreds of sites. Interpretation of the images involves readers who know how to consistently interpret the images according to certain guidelines. The number of readers also provides a potential source of variability. This means that the number of readers should be kept to a minimum.
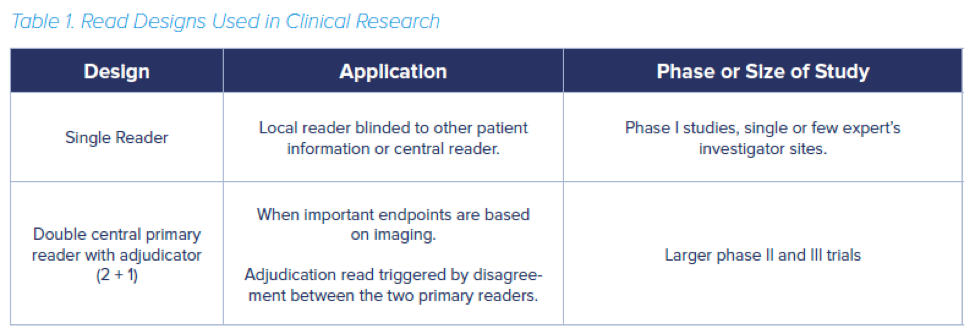
When it comes to interpreting medical images within the clinical trial context, the type of ‘read design’ used is of paramount importance for decreasing bias. There are three basic types of read designs: single read, double read and double read with adjudicator. Table 1 summarizes which types of read design tend to be used in clinical trials.
In a single read, only one radiologist will interpret the image. In a double read, two or more radiologists read the same images. Due to the workload in a large trial, multiple readers may be involved. In that case preferably one (or two in a double read design) reader(s) will review all the images from one particular patient throughout the study duration. If multiple readers review different imaging time points of the same patient additional variability may occur.
In contrast to the clinical practice setting, where a radiologist provides a narrative of a patient’s response to treatment, in a clinical trial the read must be completely independent from any clinical data (unless the read criteria specifically includes a clinical component, such as the Cheson criteria – see below). Most clinical trials use a double read design in which readers try to reach a consensus or a double reader plus an adjudicator (2+1 design). In this 2 + 1 set-up an adjudicator will review those cases in which the first two readers reached a different conclusion.
As an example, in an oncology trial, the read designs usually includes the following sessions:
- Baseline/screening of lesion selection and measurement before treatment
- Sequential lesion selection and measurement at each follow-up imaging visit
- Incremental radiological response assessment at each timepoint
Some read designs also include the following:
- A global session – where readers can review all the images from baseline to the final time point in one session
- A clinical session – where other outcome measures may be used to assess progression of disease.
Another type of read that sponsors might consider is the eligibility read. This captures a subset of data and provides a high-level assessment to determine whether potential subjects meet the study’s inclusion criteria. This is sometimes used if sites are biased by clinical data or are using a sophisticated measurement and perhaps do not have as robust understanding of the disease/modality as the central readers.
Finally, sponsors might request that the central readers confirm progressive disease. This is to ensure that participants are not taken off a study prematurely and increases the likelihood that the dataset will be complete.
Some of these read designs may require more resources than single or basic double reading, but the opportunity to highlight potential errors or bias at the time of detection in the clinical trial context is essential to ensure good data quality.