Why are so many imaging queries sent to your site and how can you avoid them?
When imaging is an endpoint in clinical trials, standardization is crucial to minimize bias and variability and to obtain robust results. One of the key steps to standardize in the image workflow is image acquisition. Imaging core labs such as Keosys will produce Image Acquisition Guidelines (IAG) to give sites guidance on how to acquire images of high enough quality for central reviews.
After image acquisition and centralization on an imaging platform, imaging technologists perform a quality control (QC) step to verify that these guidelines were correctly followed. An image that fails the QC check will be subject to a query process, which can inconvenience sites and delay image workflows.
The most common queries
The most frequent cause for QC failure is missing data. Sites can make mistakes when uploading data and can forget to select a specific series. Training sites is key to ensure that all mandatory data has been correctly acquired and uploaded.
Another frequent cause for query is non-compliance to the IAG. Incorrect acquisition parameters can have an impact on central reads; the IAG must be shared with the imaging department and read by the imaging technologists who will perform the scans. If imaging is not performed on site, the IAG should be provided to the medical imaging center that will perform imaging during the study.
The uploading of data with patient health information (PHI) present is another major cause for query. Most centralization software, such as Keosys’ proprietary technology, Imagys, have de-identification capabilities for DICOM formats. However, screenshots or reports with PHI need to be removed from the data or be manually de-identified prior to transfer.
Site training and monitoring
To limit the number of queries in your study, site training is crucial. Imaging requirements may be different from local routine procedures and can be challenged by the site. Imaging core labs like Keosys have imaging specialists who can discuss imaging protocols directly with the site’s imaging team. Together, they can find solutions to define acquisition parameters that are feasible by the site and that will produce quality images needed for the study.
We recommend involving the site’s imaging team as much as possible in communications with the imaging core lab. The person uploading images and receiving queries may not have a background in imaging and may not understand what is missing. Direct communication between the site’s imaging technologist and the imaging core lab can help quickly clarify any misunderstanding in terms of what is expected, particularly in cases where mandatory requirements are not standard practice in clinical routine.
During the study, site performance should be monitored. At Keosys, project management teams will not only monitor if images are acquired and uploaded on time but will also record the number of queries sent to the site, their frequency, and the reasons behind each query. Deviation from key performance indicators defined at the start of the study will be reported and may trigger site re-training.
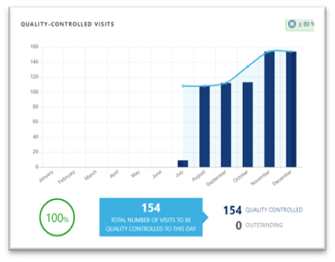
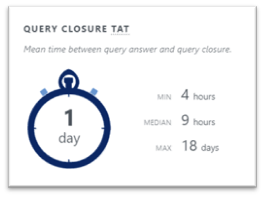
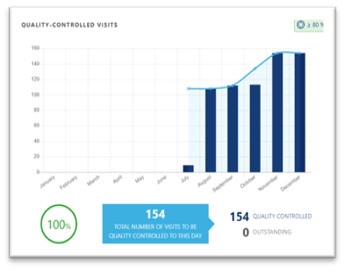

Conclusion
Images failing quality control because they are not appropriate for central review is a frequent challenge in clinical trials. As a result, not only might the sponsor lose information, but more importantly, a patient who could benefit from a treatment might not be enrolled in a trial. Working with an imaging vendor who trains and monitors sites can mitigate this risk.
Reach out to our sales team to find out more about Keosys’ services:




