Precise characterization of the kidney and kidney tumor characteristics is of outmost importance in the context of kidney cancer treatment, especially for nephron sparing surgery which requires a precise localization of the tissues to be removed.
At Keosys, we faced this challenge using AI algorithms to automatically recognize and separate the healthy kidney tissue from the cancerous one.
Kidney Tumor
Kidney cancer represents the 2.4% of the cancers worldwide with more than 400 000 new cases in 2018 with large variations in incidence rates based on geography, ethnicity, gender and over the time. In the last two decades, the growing use of medical imaging had two effects: the renal tumor size diagnosed has consistently decreased, and the number of localized renal masses found incidentally, on the other hand, has raised.
While the standard for renal cancer treatment has been traditionally radical nephrectomy (i.e. removal of both the tumor and damaged kidney), more preservative nephronsparing surgery (i.e. open or laparoscopic partial nephrectomy) has more than quadrupled in the last 20 years and is now a treatment of choice.
In this context, the information related to the kidney and tumor positions, shapes and sizes, is of great importance for the surgery evaluation and planning process. While imaging modalities such as CT allow precise detection of the tumors, there is still a lack of automatic delineation tools, and the evaluation continues to rely on the use of nephrometry scoring systems based on manual and relatively simple imaging features.
KiTS Dataset
In the last years semantic segmentation has substantially improved, establishing itself as a promising tool for the medical images pre-processing. One of the main causes that has contributed to the research and the continuous improvement of automatic segmentation techniques with the employment of deep learning algorithms, is due to the availability of public dataset on which such AI solutions can be experimented and validate.
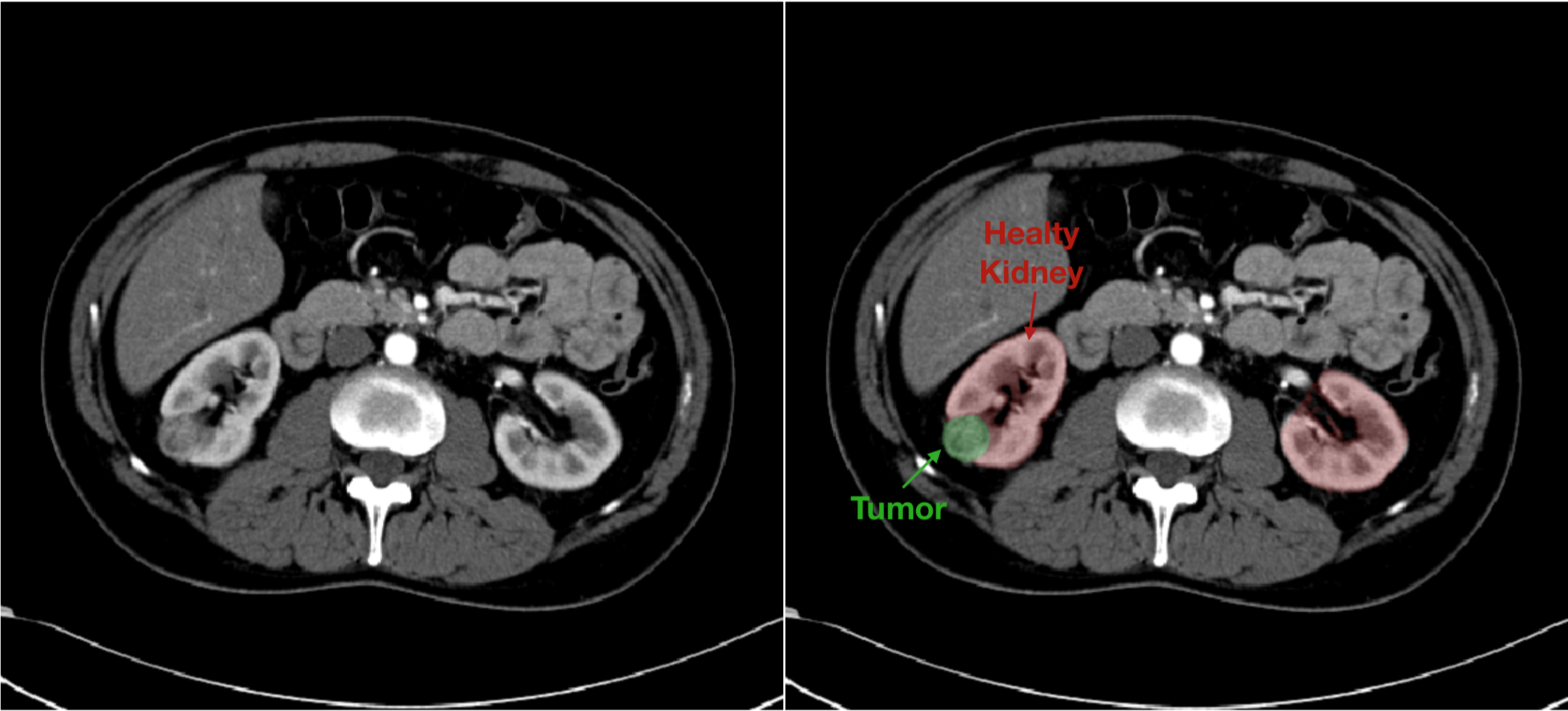
In this scenario, to accelerate research into new systems that can quickly identify renal cancer with increasingly high precision, a new public dataset was recently released: the kidney tumor segmentation dataset (KiTS) for the homonym segmentation challenge. It consisted of 300 contrast enhanced CT scans (CECT), acquired in the pre-operative arterial phase and selected from a cohort of subjects who underwent a partial or a radical nephrectomy between 2010 and 2018 at the University of Minnesota. The dataset provided also for each included case the labeled image of both tumor tissue and healthy kidney tissue (Fig 1).
Fig. 1. Example of data provided in the KiTS database. On the left the greyscale CT image. On the right the mask image is overlapped to native image in order to highlight the component of interest.
Our Solution
To solve this segmentation challenge we developed a multi-stage segmentation approach as reported in Fig. 2.
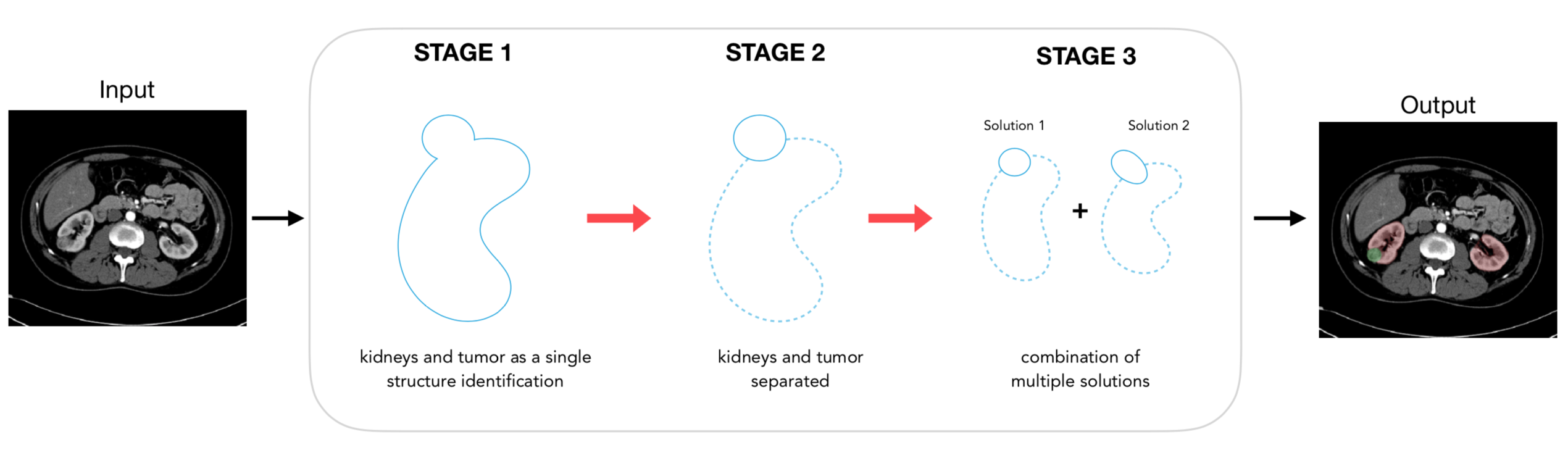
Fig. 2. Schematic representation of the system designed to automatically identify and separate the healthy kidney tissue and the tumor. In stage 2 and 3 the dotted line represents the kidney while the continuous line identifies the tumor.
The first part aims to roughly segment the region of interest (ROI) where to focalize the subsequent analysis, in this case the kidney region. In the second stage, the segmentation of the kidneys and cancerous tissue is carried out by two different convolutional neural networks (CNNs), which work on the image sub-portions extracted using the approximate kidney predictions from stage one. The results are finally combined in the last stage, where the final segmentation is obtained by using an ensembling operation.
The segmentation quality was evaluated by using the Dice score coefficient, which measures the agreement between the automatic segmentation and the manually defined ground truth by calculating the overlapping area between the two segmentation masks.
We trained our algorithm on almost 200 cases and we tested it on 90 cases never seen before, in order to assess the robustness and generalization capability of our system to perform well on new cases.

With our configuration we obtained very good results in terms of kidney tissue segmentation (dice score 0.96) and promising ones in terms of tumor detection and delineation (dice score 0.74).
A comparison of our segmentation system on same random cases is shown below together with manual references provided in the KiTS dataset.
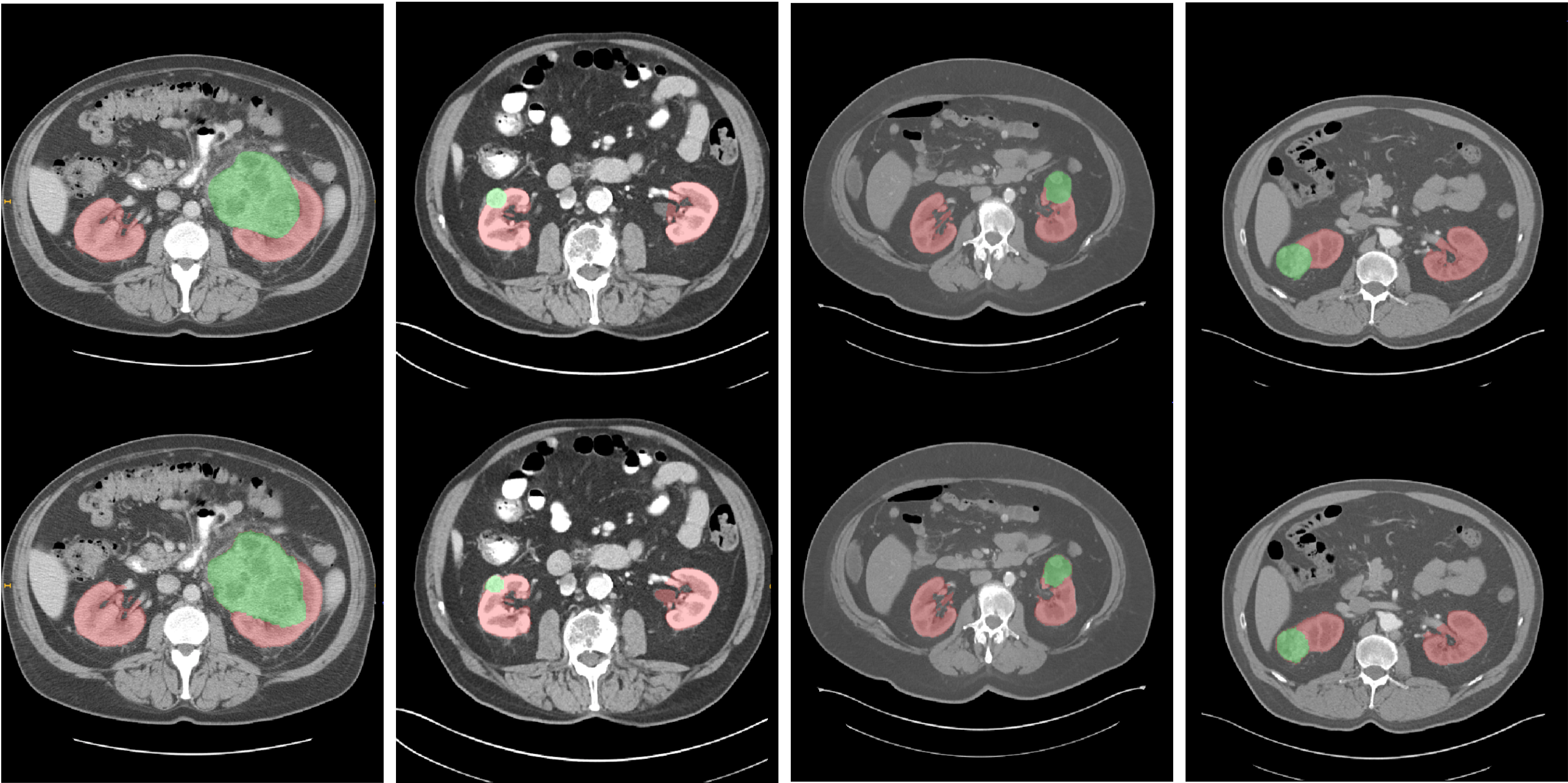
Fig. 3. Segmentation examples from four patients. On the top row the ground truth labels are reported (red for kidneys, green for tumors). The proposed model predictions are on the second row. Best viewed in color.