This is an excerpt from our free eBook, “Hepatocellular Carcinoma and Imaging in Clinical Trials.” To access the full eBook, click here.
In oncology clinical trials, response to treatment is generally evaluated by assessing changes in tumor size. Response Evaluation Criteria In Solid Tumors (RECIST 1.1)[6] is the international standard criteria most commonly used. Readers select at baseline lesions to represent tumor burden: Target Lesions and Non-Target Lesions. The selected lesions are then evaluated at each time point to assess change in tumor burden over time.
- Five Target Lesions are measured, and the sum of their longest diameters (SOD) is calculated at every time point.
- Non-Target Lesions are evaluated qualitatively.
When it comes to HCC, liver-specific challenges exist (e.g., tumor devascularization) that RECIST 1.1 does not consider which can result in an underestimation of tumor response. Indeed, locoregional intra-arterial therapies or molecular targeting therapies lead to early tumor necrosis or intratumoral changes and delayed tumor shrinkage. In addition, coexistence of cancer and cirrhosis can lead to possible misinterpretation of certain manifestations. For example, liver changes caused by cirrhosis may mimic or conceal intrahepatic tumors. With chronic liver disease there can also be a development of ascites or an enlargement of the lymph node at the porta hepatis that with RECIST 1.1 would be assumed to represent evidence of tumor progression with RECIST 1.1.
Llovet JM and Lencioni R therefore proposed a modified version of RECIST (mRECIST), adapting RECIST 1.1 to the particularities of HCC[7]. The notion of “viable tumor” was introduced as the portion of the tumor that shows contrast enhancement on arterial phase. When assessing a lesion, only the “viable tumor” is measured, thus excluding non-enhanced areas from the measurement (Figure 3).
Figure 3: Measurements of different viable tumors as per mRECIST
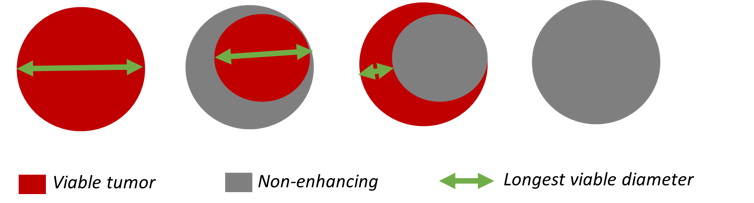
mRECIST guidelines are currently the most widely used criteria to evaluate tumor response to treatment in patients with HCC. The principles used to determine tumor response are largely unchanged from RECIST 1.1. mRECIST, however, introduces specific rules for the selection and measurement of intrahepatic lesions and gives specific guidance for the assessment of lymph nodes, ascites, portal vein thrombosis, and newly detected hepatic nodules.
Table 2: mRECIST compared to RECIST 1.1
| RECIST 1.1 | mRECIST | |
| Lesion selection | Up to 5 Target Lesions (2 max per organ) to measure and Non-Target Lesions to assess qualitatively |
Same as RECIST 1.1 + specific definitions for HCC lesions:
Typical intrahepatic lesions must be prioritized over atypical intrahepatic lesions. |
| Specific lesions | N/A |
|
| Target Lesion measurement | Nodal lesion: SA Non-nodal lesion: LD |
|
| Sum of diameters | Sum of all Target Lesion measurements | Same as RECIST 1.1 but sum can include measurements of LVD as well as LD and SA. |
| New Lesion | Appearance of new unequivocal lesions | New hepatic lesion: LD ≥10mm and showing arterial phase non-rim-like hyperenhancement + non-peripheral washout in the portal venous or the delayed phase. |
| Complete Response |
|
|
LD: Longest Diameter; SA: Short Axis; LVD: Longest Viable Diameter
Other criteria can be used to evaluate tumor response in HCC as described in Evaluation of liver tumor response by imaging by Gregory et al.[8] such as the EASL criteria, the Choi criteria, and the LI-RADS treatment response algorithm. 3D-based criteria have also been developed.
EASL criteria was developed prior to mRECIST. The main difference between the two is that the EASL criteria, dating back from before the introduction of RECIST and the concept of unidimensional measurements, measures the product of the longest diameters of the viable portion of the tumor. Consequently, thresholds for partial response (PR) and progressive disease (PD) are different with, respectively, ≥50% decrease in total tumor load of all measurable lesion(s) and ≥25% increase in size of one or more measurable lesion(s). EASL criteria are also only used for the evaluation of Target Lesions and are not applicable in the context of systemic treatments for HCC.
The Choi criteria was initially developed for the management of patients with advanced metastatic Gastrointestinal Stromal Tumor (GIST) treated with imatinib (a tyrosine kinase inhibitor). Even though no changes in tumor size were seen, patient response to treatment was still observed (Choi et al)[9], therefore proposing a criteria where both size and CT attenuation were evaluated. As imatinib and sorafenib have similar biological effects in terms of reduction of vascular cancer feeding, the Choi criteria was studied with HCC. Treatment response defined by the Choi criteria was associated with longer survival in patients with advanced HCC treated with sorafenib[10].
The LI-RADS also comprises an algorithm for the assessment of HCC treated with local-regional therapy. Lesions are categorized as LR-TR Nonviable, Equivocal, or Viable according to their imaging features (e.g., focal hyperenhancement, washout, etc.). The algorithm gives an individual tumor status instead of an overall patient level response[11].
2D-based criteria present several limitations as HCC tumors tend to grow asymmetrically with different areas changing size while the longest diameter remains unchanged. In addition, measurement methods are limited by high inter-and-intra-reader variability. 3D quantification based on automatic and semi-automatic segmentations can provide reliable and reproducible data to evaluate treatment response. Tumor changes are detected earlier, thus improving the prediction of overall survival.
In a recent interview with Keosys, Maxime Ronot, Professor at the Université de Paris, and Radiologist in the Department of Medical Imaging at the Beaujon University Hospital in Clichy, France, shared his thoughts on the read criteria used in HCC: “Our problem is that we see there is more and more discrepancy between the way the tumor behaves and the outcome of patients, especially when you use immunotherapy. You can have stable disease for months and months, or some patients will progress, but you stick with the treatment and in the end, it will benefit the patients. I am not sure that iRECIST, the criteria that are designed for immunotherapy, are going to be really useful in HCC, because the occurrence of pseudo progression or hyper progression seems to be extremely rare. So, probably the benefit of those criteria is not needed in HCC patients. Maybe that's a possible simplification in the years to come. But we will keep the mRECIST part, I guess, because we still use molecules that target vascularization of tumors. So, I think this one is going to stay.”




