We previously introduced the concept of imaging read designs in clinical trials. In that post, we provided points to consider in ensuring that the number, type of readers and the setup used to capture and interpret the imaging data are optimized. Here, we explore the topic in a bit more detail, using the FDA’s Guidance on “Clinical Trial Imaging Endpoint Process Standards” as a basis.
Definition of Terms
First, we provide definitions of the terms commonly used in the field. This is to ensure clarity, as different terms are used in different contexts, research papers and guidance documents.
Local read: Images captured and read at a specific clinical trial/imaging site. Clinical trials often take place at multiple centers. As such, each investigational site provides ‘local’ reads of the images acquired at that site. The site reader may or may not be aware of the treatment given to the participant.
Central read: Images acquired at various clinical sites are sent to a ‘central’ site for independent review and quality assurance. This step is also referred to as “blinded independent central review” (BICR), whereby ‘blinded’ means that the reader does not know what treatment the participant is given. This step aims to reduce any bias in image interpretation. The ‘imaging core lab’ is often used to perform central reads.
Double-read with adjudication: The adoption of two central reads provides a mechanism to ensure that image interpretation is robust and that there is precise assessment of treatment effect (double-read). However, if consensus is not reached, a third reader is used to ‘adjudicate’ the decision. The rate of adjudication required for a trial has become a useful metric to assess the performance of a trial. That is, low rates of adjudication can indicate good compliance with the study protocol. The variability among central readers can also be similarly assessed.
Pivotal trials: clinical trials that are ‘pivotal’ for marketing authorization/approval by the regulatory authorities. As such, they are also sometimes called ‘registration(al)’ trials. Typically, pivotal trials are Phase III trials—the stage at which the efficacy of an investigational therapeutic (or a medical device) is tested in larger and more diverse patient populations. However, given recent innovations in trial design, therapeutics tested and regulatory pathways for approval, Phase II trials may also be considered pivotal. If the trial shows that the investigational therapeutic is safe and effective, then the data from that trial form a large part of the dossier given to the regulatory authorities for review for approval.
Selecting the Appropriate Read Design for Your Trial
The FDA’s imaging endpoint guidance states: “The usefulness of a centralized image interpretation process is determined by the role, variability, and susceptibility to bias of imaging within the trial as well as modality-specific image quality considerations and overall trial design features.”
That is, selection of the read design and the frequency and timelines of image interpretation will depend on the trial design itself. The guidance highlights several advantages of implementing centralized image interpretation, including “more verifiable and uniform reader training as well as ongoing management of reader performance”. However, it also takes into account instances when BICR might not be necessary:
“Centralized image interpretation is not always critical, even for a phase 3 trial primary endpoint that uses some aspects of quantitative imaging, if the quantitative measures are widely performed and reported in clinical medicine, little imaging acquisition or interpretation variability is anticipated, and potential biases in image interpretation are controlled by the trial design features.”
Much research is being devoted to assessing the strengths and limitations of various read designs for different indications. For example, one study advocates a 2+1 read methodology for ulcerative colitis clinical trials. In cardiology, it is noted that when site-reported rather than core laboratory-adjudicated imaging results are to be used, the skill of the interpreting physicians at the local sites must be taken into consideration.
Meanwhile, in oncology, one group suggested that when double-blinded trials are not feasible for assessing progression-free survival (PFS), BICR is not recommended as a general strategy for reducing bias. Instead, BICR would serve a more useful purpose as an auditing tool to assess biases at the local-read level. However, another study noted that BICR of PFS can be a cost-effective strategy as it can decrease sample sizes and trial costs – an important factor considering the substantial costs involved in running a pivotal trial.
The overarching goal of all these endeavors is to minimize bias and variability. To this end, advancements in image analysis technology and software, such as our Read System, for improving the accuracy and speed of the image evaluation process are contributing to such efforts.
We have the expertise and flexible approach to support your decision-making with regard to which read design is optimal for your trial.
Key Benefits of Imaging in Clinical Trials
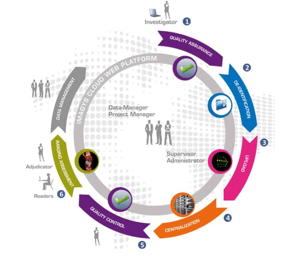
Keosys’s IMAGYS platform can manage every step of the clinical trials process: from image acquisition at an investigator site to data submission to the regulatory authorities. Our products enable all stakeholders to stay connected and informed throughout the trial on a global scale.




