This is an excerpt from Keosys' free eBook, "An Introduction to Peptide Receptor Radionuclide Therapy." To access the full eBook, click here.
Peptide receptor radionuclide therapy (PRRT) is a form of targeted cancer therapy. It consists of a small peptide, or a peptide analog, that is coupled to a radionuclide. In this way, PRRTs are examples of radiopharmaceuticals – pharmaceutical agents that are coupled to a source of tumor-destroying radioactivity.
The peptide part of the molecule specifically binds to cell-surface proteins that are overexpressed in certain cancer types. Examples of these proteins include somatostatin receptors that are over-expressed in neuroendocrine tumors and prostate-specific membrane antigen (PSMA), which is expressed in prostate cancer.
This targeted form of systemic radiotherapy enables tumor-destroying radionuclides to be delivered directly to cancer cells. The radionucleotides are beta emitters; in particular iodine-131, lutetium-177 and yttrium-90. Beta emitters offer the advantage of a very small range of iradiation (<1.6mm for 177Lu), so there is less risk of damage to surround healthy tissues.
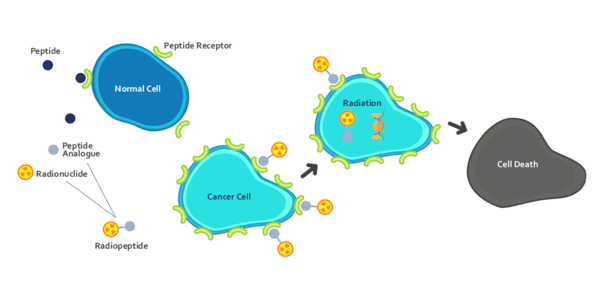
When the peptide binds to the cell surface receptor, the receptor is internalized and hence the radioactivity is delivered directly into the intracellular space of the tumor cell. This retention of intracellular radiation is associated with DNA damage through beta-emission and subsequent cell death. This mechanism of action (Figure 1) means that PRRT is not susceptible to the cellular resistance mechanism often observed with conventional chemotherapy.
PRRT has been in use been since mid-1990s in a handful of centers in Europe (in patients with individual therapy plans). Formal, larger scale clinical trials began in 2013, which ultimately led to the regulatory approval of a first in class PRRT: Lutathera (lutetium-177 dotatate). This gained EU approval in 2017 and US approval in 2018 for the treatment of neuroendocrine tumors.
Figure 1 - PRRT Mechanism of Action
Conditions That Benefit From PRRT
PRRT can be used for tumors that express peptides that are not found on normal cells, or are found in higher levels on tumor cells compared to normal cells. A key benefit of PRRT — compared to other treatments such as surgery or chemotherapy — is that the peptide targeting mechanism enables the radionucleotide therapy to accesses disease at the cellular level. Two examples of tumors that can be treated with PRRT are neuroendocrine tumors and prostate cancer.
Neuroendocrine Tumors
Neuroendocrine tumors (NETs) are a relatively rare form of cancer that arise from neuroendocrine cells. These cells receive signals from the nervous system and respond by releasing hormones to perform specific functions. Because the neuroendocrine system is distributed throughout the body, NETs can develop anywhere in the body, including the stomach, intestines, pancreas and lungs.
The majority of NETs (60-70%) originate in the gastro-entero-pancreatic tract (GEPNETs). This subtype expresses high levels of somatostatin receptors, including subtype 2 (sst2) and subtype 5 (sst5). GEP-NETs is the indication for which PRRT is most clinically well developed. Some NETs arise from respiratory neuroendocrine cells; these are referred to as broncho-pulmonary (BP)-NETs. A high proportion (about two-thirds) of bronchial NETs over-expresses somatostatin receptors, especially the sst2 subtype.
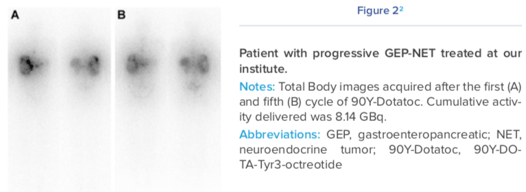
NETs can be tricky to detect. They are very small, can remain clinically silent for years, and unlike many other tumors, NETs do not rapidly consume glucose, so standard PET scans using fludeoxyglucose cannot usually be used for detection. This means they are often quite advanced when detected.
Prostate Cancer
Many prostate cancers, in particular those that have spread or become resistant to hormonal therapies, express an antigen on their cell surface called prostate-specific membrane antigen (PSMA); this is an enzyme that is also referred to as glutamate carboxypeptidase II3. The level of PMSA correlates with a number of important metrics of tumor aggressiveness, the propensity to metastasize and the development of disease progression despite androgen depletion therapy.
The development of 177Lu-labeled small ligands for prostate cancer was triggered by the need for effective metastatic castration-resistant prostate cancer therapy and by the success of PRRT in patients with metastatic neuroendocrine tumors. Importantly, PRRT targets bone metastases in prostate cancer, which accounts for severe morbidity in a large proportion of patients that are resistant to androgen depletion therapy.