An earlier post provided a broad overview of the various imaging criteria that are used for evaluating treatment responses. RECIST1.1 are the most commonly applied in oncology clinical trials. However, it is widely agreed that different cancer types require tailored criteria to ensure accurate determination of treatment responses.
In this post, we focus on RANO (Response Assessment Neuro-Oncology) Criteria, specifically for glioblastomas.
What are Glioblastomas?
Glioblastomas (also known as grade 4 gliomas/astrocytomas) are rare but often deadly brain tumors that primarily occur in older people (median age of diagnosis of 64 years). The incidence rate is 3.19 per 100,000 persons in the United States and less than 5% of patients survive 5 years following diagnosis.
Treatment usually involves resection of the tumor and then radiotherapy plus several rounds of the chemotherapeutic temozolomide. Even with treatment, however, the median survival time is about 14.6 months; without treatment, survival is about 3 months.
Clearly there is an acute need for better treatments for glioblastomas. However, the complex pathobiology of this type of cancer, among other factors, is proving challenging for drug developers. In parallel, accurately analyzing treatment responses is complex. Imaging of tumor size and vascularization is playing a pivotal role in addressing this aspect.
Standardized methods for assessing responses (i.e., surrogate endpoints) to glioblastoma therapies are available, and the RANO Criteria are the most widely applied in glioblastoma clinical trials. However, this is likely to change in the future, as discussed below.
CT and MRI are the main imaging modalities used for diagnosing and assessing treatment responses for glioblastoma. For a long time though, there were no standard guidelines regarding how responses were assessed for brain tumors.
This all changed in 1990, when David Macdonald and colleagues published a paper presenting new uniform response criteria for Phase 2 studies of supratentorial malignant glioma.
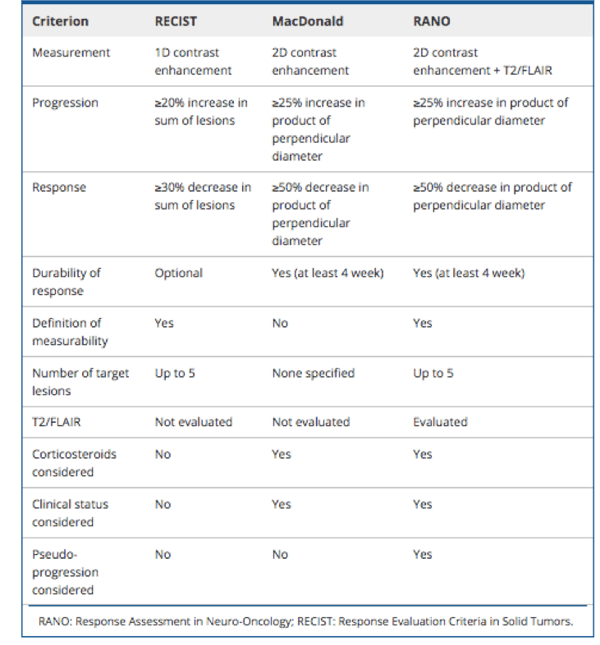
These criteria (summarized in Table 1) use changes in 2D contrast enhancement tumor measurements to categorize the following four responses: complete response, partial response, stable disease, and progressive disease.
The ‘Macdonald Criteria’ were widely used in clinical trials and enabled robust comparisons among trial data. However, limitations of these criteria became particularly apparent in the advent of anti-angiogenic therapies and the phenomenon of pseudoprogression in patients receiving chemoradiotherapy.
Development of RANO
According to the Macdonald Criteria, a ≥ 25% increase in the contrast-enhanced image is a marker for tumor progression. However, contrast enhancement is nonspecific and might be due to factors such as anti-angiogenic agents or changes in radiologic techniques.
Experience with the anti-angiogenic agent bevacizumab highlighted limitations of the Macdonald Criteria. Bevacizumab can cause reduced contrast enhancement, contributing to high response rates and prolonged progression-free survival. However, overall survival is not extended and tumor size is not reduced; a phenomenon termed pseudoresponse. Moreover, non-enhancing tumor progression is not considered in the Macdonald Criteria; consequently, disease progression might be missed.
In 2010, the RANO Criteria (summarized in Table 1), which address these limitations, were published. An important update was the inclusion of MRI T2-weighted and fluid-attenuated inversion recovery (FLAIR) imaging to monitor non-enhancing disease progression.
How the RANO Criteria are Used in Clinical Trials
In addition to recognizing the phenomena of pseudoresponses, pseudoprogression and non-enhancing progression, the RANO Criteria clarified the criteria for patient eligibility for enrollment into clinical trials.
In the past, patients showing even minimal changes in imaging parameters that indicated worsening of disease were eligible for inclusion in clinical trials for recurrent gliomas. Among other features, the RANO Criteria now specify “a 25% increase in the sum of the products of perpendicular diameters of the contrast-enhancing lesions, while on stable or increasing doses of corticosteroids, before they are considered to have progressive disease and are entered onto clinical trials for recurrent/progressive disease”.
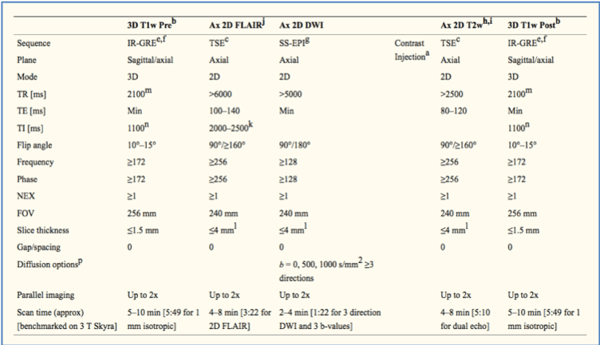
This clarification helps ensure that the right patients are selected for enrollment. The RANO Criteria are now the response criteria used in neuro-oncology, and were considered in the development a standardized Brain Tumor Imaging Protocol in clinical trials (see Table 2 for an example MRI protocol).
Keosys has provided integral support and are supporting trials that adopted the RANO Criteria, from Phase 1 to Phase 3.
The Future of the RANO Criteria
Although a welcome improvement to the Macdonald Criteria, the RANO Criteria are still very much a work in progress. Indeed, subgroups of the RANO Criteria are under development (e.g., iRANO, RAPNO, RANO-BM and) to help refine surrogate endpoints and enable objective assessments of specific types of therapies (e.g., immunotherapies vs anti-angiogenics), patient groups (e.g., pediatric vs adults) and disease type (metastatic vs primary).
In this dynamic field, our experience and expertise can guide decision-making processes to ensure that oncologists and radiologists have a solid understanding of the RANO guidelines, including their limitations, to achieve accurate assessments of responses to therapeutics.